
Internal Medicine
Vaccination on patients on immunosuppressive medications and chemotherapy
Deciding whether to vaccinate an animal on immunosuppressive medication or chemotherapy is challenging. Vaccination in such patients is not sanctioned by vaccine companies, as they have not thoroughly investigated their products in this setting. There is some experimental data to offer guidance in these situations, but this is limited. As both pet owners and vets, we are keen to avoid preventable diseases, but do not want to cause harm by vaccinating animals if it is unsafe to do so. Ultimately the decision to vaccinate any animal on immunsuppressive medication or chemotherapy must be made on an individual basis, by considering their vaccinal history, their current health status and medications, and their current risk factors for exposure to these infectious diseases.
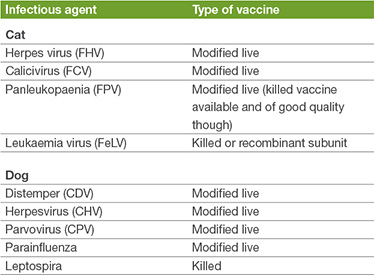
The aim of vaccination is to prevent or reduce the impact of infectious disease in an individual patient and in the population beyond (herd immunity). Adequate response to vaccination requires a response from the patient’s immune system. Patients with immune compromise as a result of illness or medication may not generate an appropriate immune response to vaccination, thus the vaccine may fail to induce protection. When modified live vaccines are used, there is also the capacity for the vaccinal agent to cause disease in an immunocompromised patient. While killed / inactivated vaccines avoid this problem, they still require a competent immune system to generate an immune response, they generally require multiple doses to be given and an adjuvant within the vaccine formulation to increase their potency. There are relatively few killed vaccine options of good quality for dogs and cats. Modified live vaccines are standard for most core vaccines.
How long will the immunity from historic vaccines last?
The immunity from CDV, CHV and CPV is prolonged, and based on both challenge studies and serology, is expected to be more than 5 years. Leptospira vaccines however provide relatively short-term immunity and annual booster vaccination is recommended in ordinary circumstances.
The immunity from FPV is also long-lasting (more than 7 years). Assessment of the degree of residual immunity after FHV and FCV vaccination is difficult to estimate, as neither vaccine provides the same robust degree and duration of immunity as FPV. Antibody titres persist for more than 3 years, but do not correlate well with the degree of protection offered.
The datasheet supplied with each vaccine product will give details of the recommended revaccination intervals, for use in regular circumstances. We have seen some changes in recent years, to align datasheets with available experimental data, and some vaccines which were historically recommended for annual revaccination are now licensed for triennial revaccination (CDV/ CHV/ CHP for example). It can be appreciated thus that the datasheet takes a conservative approach on the recommended revaccination intervals, as it is key that animals vaccinated with the product do not succumb to disease. In many instances however, post-vaccinal immunity will be much longer-lasting than is suggested by the recommended revaccination interval on the datasheet.
Vaccine products can only be licensed for use in the situations in which they have been thoroughly trialled. Thus datasheets generally cannot advocate vaccination of animals receiving immunosuppressive/ cytotoxic medications.
There are relatively few experimental studies assessing the impact of immunosuppressive medications or chemotherapy on vaccine response in veterinary patients, however some data do exist:
- Studies in both cats and dogs suggest that immunosuppressive steroid treatment prior to or concurrently with vaccination does not significantly impair vaccinal antibody production in naïve dogs or in the booster vaccination setting.
- A study of cats on ciclosporin showed that antibody production in response to booster FPV and FCV revaccination was unaltered, but the serological response to FHV and FeLV booster revaccination were slowed. There was inadequate antibody production in ciclosporin-treated cats when vaccinated with a primary course of FIV vaccine. The suggestion is that ciclosporin impairs primary but not memory vaccinal immune responses.
- Several studies looking at vaccinal responses in animals receiving chemotherapy show adequate immune responses in these patients.
The decision to vaccinate an animal on immunosuppressives /chemotherapy should be based upon the animal’s previous vaccinal history and the current perceived risk from the infectious agents under consideration.
Can I monitor immunity with antibody titres?
Documentation of the presence of serum antibody levels for CDV, CAV, CPV and FPV to determine the immunity status is well established. In some countries, this is routinely performed at 3-yearly intervals (instead of automatic revaccination for core diseases). Assessment of serum antibody levels to FCV and FHV can be measured, but do not correlate well with clinical protection, so is not advised. It is well known that antibody levels for Leptospira do not correlate with the degree of immunity, thus testing Leptospira antibodies is not advised for the purpose of investigating vaccinal protection levels.
Is there a capacity for vaccination to exacerbate previously-diagnosed immune-mediated disease?
Although vaccines are not anticipated to cause auto-immune disease, in genetically-predisposed animals, any immune stimulus (disease, infection, medication etc) can potentially trigger autoimmunity. When considering auto-immune disease, very few cases are reported in which vaccination may have been a trigger. In an individual animal where vaccination is suspected to be an inciting trigger for its immune-mediated disease, careful consideration of the animal’s vaccinal history, antibody levels and lifestyle risks should be undertaken to decide upon the risk/benefit status of revaccination.
What do the WSAVA guidelines say?
WSAVA recommends against vaccinating pets on immunosuppressive or cytotoxic therapy (other than glucocorticoids). The rationale for avoidance of modified live vaccines in these patients is the potential for such a vaccine to cause disease. Killed vaccines are not advocated, as many are ineffective. WSAVA also recommends waiting at least 2 weeks after stopping immunosuppressive therapy before revaccinating a pet.
- Day, M.J., Horzinek, M.C., Schultz, R.D., Squires, R.A. Guidelines for the vaccination of dogs and cats. Complied by the vaccination guidelines group (VCG) of the world small animal veterinary association (WSAVA). 2016. Journal of Small Animal Practice, 57 (1), pages E1-45.
- Roberts, E.S., VanLare, K.A., Roycroft, L.M., King, S. Effect of high-dose ciclosporin on the immune response to primary and booster vaccination in immunocompetent cats. 2015. Journal of feline medicine and surgery, 17 (2), pages 101-109.
- Gershwin, L.J. Adverse reactions to vaccination; from anaphylaxis to autoimmunity. 2018. Veterinary clinics of North America: Small Animal Practice, 48, pages 279-290.
- Walter, C.U., Biller, B.J., Lana, S.E., Bachand, A.M., and Dow, S.W. Effects of Chemotherapy on Immune Responses in Dogs with Cancer. 2006. Journal of Veterinary Internal Medicine, 20, pages 342–347.
Case Advice or Arranging a Referral
If you are a veterinary professional and would like to discuss a case with one of our team, or require pre-referral advice about a patient, please call 01883 741449. Alternatively, to refer a case, please use the online referral form