
Tyrosine and kinase are two words that many of us hoped we would never have to hear again once preclinical veterinary training was complete. However, the rapid developments in 21st century medicine have led to the creation of completely new categories of medicines.
For us, the tyrosine kinase inhibitors are the first in this era of new pharmaceuticals. Be prepared to dust off your molecular biology textbooks; the future looks bright, albeit a little complicated.
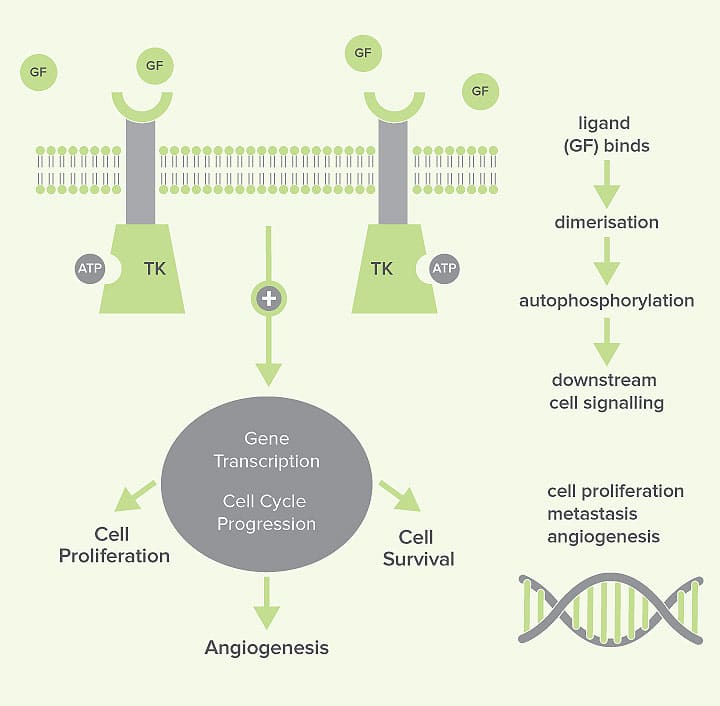
Receptors with tyrosine kinase enzymes in their active site (we call them receptor tyrosine kinases) are key players in normal cell signalling. They participate in regulation of cell growth and differentiation.
The tyrosine kinase elements bind to ATP and then use this to add phosphate groups onto themselves (‘autophosphorylation’). This initiates an intracellular signalling pathway that impacts cell proliferation and survival. The autophosphorylation process usually occurs in response to an external signal, for example, the binding of a growth factor.
Receptor tyrosine kinases can be located at the cell surface, in the cytoplasm or in the nucleus. Examples of receptor tyrosine kinases that you may have heard of include Her 2/neu and c-kit. Her 2/neu is associated with certain forms of breast cancer in humans; c-kit has risen to prominence in the veterinary world due to its association with mast cell tumours. These molecules are predominantly associated with cell proliferation functions. In addition, certain receptor tyrosine kinases are important in tumour angiogenesis, for example, VEGFR (vascular endothelial growth factor receptor).
Tyrosine Kinases in Cancer
In malignant tumours in both human and veterinary patients, tyrosine kinases are often abnormally activated. The consequence is persistent signalling in the downstream pathways, resulting in uncontrolled cell proliferation and survival (see figure 1).
Although tyrosine kinase dysfunction has been extensively characterised in human oncology, it is far less studied in veterinary oncology. Mutations in c-kit have been identified in canine mast cell tumours and gastrointestinal stromal tumours. In the majority of affected dogs, the mutations consist of in-frame (whole codon) DNA duplications causing changes in the receptor molecule just inside the plasma membrane.
Such mutations result in constant activation of the c-kit molecule in the absence of a growth factor trigger.
Approximately 15% of all canine mast cell tumours carry c-kit mutations. There is an association between presence of a mutation and tumour grade. Mutations are rarely identified in well-differentiated tumours while they are reported in up to 65% of poorly-differentiated mast cell tumours.
Even within the histological categorisation that is poorly-differentiated (Patnaik grade 3) mast cell tumours, there is still a significant amount of variation in clinical evidence of malignancy. It is reasonable to assume that the likelihood of c-kit mutation increases progressively in conjunction with other markers of malignancy such as rapid growth, ulceration, etc.
What about cats?
Mutations in c-kit have been identified in feline mast cell tumours. The pattern of mutations differs significantly from that seen in canine mast cell tumours. At present it appears that mutation of c-kit is unrelated to biological behaviour.
Tyrosine Kinase Inhibitors in Veterinary Medicine
Tyrosine kinase inhibitors (TKIs) are wholly synthetic drugs, designed to block the ATP-binding site of kinases, essentially acting as competitive inhibitors. In the absence of ATP-binding, the kinase is not able to initiate the downstream signalling pathway (see figure 3).
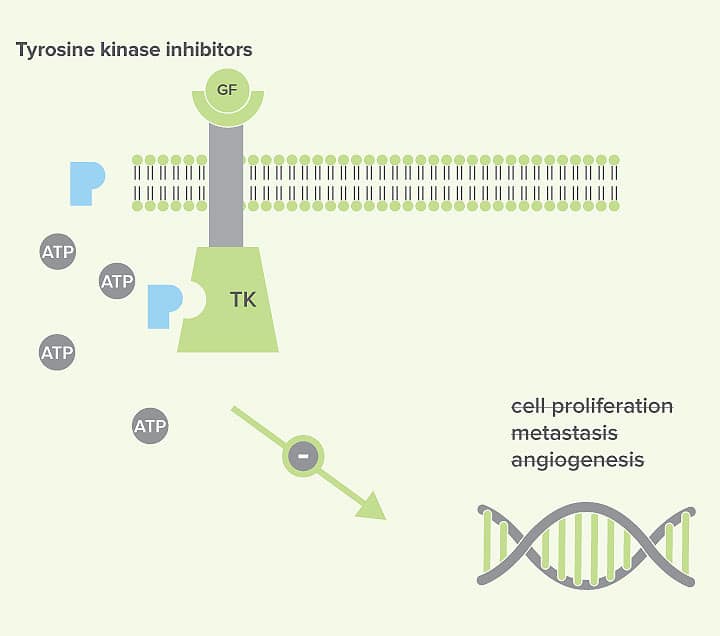
Licensed Veterinary TKIs
There are two licensed veterinary TKIs, Masivet (masitinib) and Palladia (toceranib).

The principal differences between these two agents is one of specificity as depicted in ‘Kinome Plots’ (see figure 5 and 6)
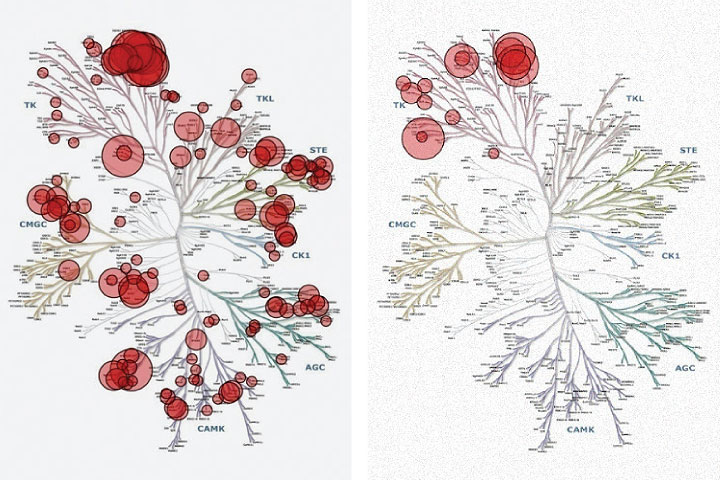
These plots demonstrate the range of known tyrosine kinase enzymes, separated according to molecular homology. The red circles indicate which enzymes are inhibited and how strongly.
Without experience of interpreting these diagrams, one can readily see that toceranib exhibits potent inhibitory activity against many receptor tyrosine kinases whereas masitinib’s inhibitory activity is more focused.
For veterinary clinicians, this could be interpreted to mean that Masivet is most likely to work in the most malignant tumours. Palladia, by contrast, would be expected to work in a proportion of other cases too because of its interactions with other targets which could also be relevant to mast cell tumour progression. This is borne out by the initial studies of these treatments.
It is also true that Masivet has a much lower likelihood of inducing significant side effects. This is similarly attributable to the more widespread activity of Palladia on other ‘non-target’ molecules. This illustration shows a simplified application. These differences influence decision-making in treatment selection.
Toxicity
Similar to chemotherapeutics, TKIs can induce toxicities that target normal tissues, likely because of the effect of chronic inhibition of receptors expressed in normal cells. These effects are more severe with TKIs that target multiple types of receptors (such as toceranib) compared with those with a narrow spectrum of kinase inhibition (such as masitinib).
Side effects reported with veterinary TKIs:
- Gastro-intestinal toxicity: anorexia, vomiting, diarrhoea, GI bleeding
- Neutropenia (more insidious in onset than associated with chemotherapy)
- Elevation of serum liver enzyme concentration
- Muscle cramping, epistaxis, hypertension, severe gastrointestinal haemorrhage and protein losing nephropathy (Palladia)
- Protein losing nephropathy and haemolytic anaemia (Masivet)
Owners must be strenuously advised to report any potential adverse effects promptly. If a treatment-associated side effect is noted, in the absence of advice to the contrary, prompt treatment withdrawal is recommended.
Monitoring
Similar to chemotherapeutics, monitoring is recommended so that adverse effects of therapy can be identified early and appropriate modifications to therapy can be applied. The following monitoring strategy would be appropriate for most patients receiving veterinary TKIs:
- Baseline body weight, haematology, biochemistry, UA, UPC, BP
- 4 weeks after starting drug recheck:
- Body Weight
- Haematology
Every 4-6 weeks thereafter recheck:
- Body Weight
- Haematology
- Biochemistry
- UA/UPC – BP
Key messages
- c-kit mutations are instrumental in the development of a proportion of canine mast cell tumours; mutations are rare in lower-grade tumours and prevalent in higher-grade tumours
- c-kit can be mutated in feline mast cell tumours but those mutations are not related to cancer development
- Two licensed tyrosine kinase inhibitors are available for treatment of canine mast cell tumours. Palladia is more broad-spectrum; Masivet is more focused
- Tyrosine kinase inhibitors do induce side effects. At worst these can be lethal. Appropriate measures must be taken to prevent and manage treatment-related adverse effects.
Case Advice or Arranging a Referral
If you are a veterinary professional and would like to discuss a case with one of our team, or require pre-referral advice about a patient, please call 01883 741449. Alternatively, to refer a case, please use the online referral form
About The Discipline
Oncology
Need case advice or have any questions?
If you have any questions or would like advice on a case please call our dedicated vet line on 01883 741449 and ask to speak to one of our Oncology team.
Advice is freely available, even if the case cannot be referred.
Oncology Team
Our Oncology Team offer a caring, multi-disciplinary approach to all medical and surgical conditions.